45+ pages 4 g of hydrogen reacts with 20g of oxygen 2.1mb. 4 g 32 g 36 g When 4 g of H 2 reacts with 32 g of O 2 gives 36 g of H 2 O. 4 g 32 g 36 g When 4 g of H 2 reacts with 32 g of O 2 gives 36 g of H 2 O. This is answered comprehensively here. Read also reacts and understand more manual guide in 4 g of hydrogen reacts with 20g of oxygen 2H_2Sg 3O_2g rightarrow 2H_2Ol 2SO_2g If 9 moles of oxygen react The reaction consumes moles of hydrogen sulfideThe reaction produces moles of water and moles of sulfur dioxide.
10 g hydrogen is reacted with 64 g of oxygen. Step by step solution by experts to help you in doubt clearance scoring.

10 G Of Hygrogen And 64 G Of Oxygen Were Filled In A Steel Vessel And Exploded Amount Of Water
| Title: 10 G Of Hygrogen And 64 G Of Oxygen Were Filled In A Steel Vessel And Exploded Amount Of Water |
| Format: PDF |
| Number of Pages: 271 pages 4 G Of Hydrogen Reacts With 20g Of Oxygen |
| Publication Date: September 2018 |
| File Size: 1.3mb |
| Read 10 G Of Hygrogen And 64 G Of Oxygen Were Filled In A Steel Vessel And Exploded Amount Of Water |
 |
2 H 2 O 2 2 H 2 O.

The mass of water formed is 1 24 g 2 36 g 3 2259 4 40g Ask for details. Now present oxygen is 20 g So O 2 will be the limiting reagent and H 2 O will be calculated from O 2 32 g of O 2 given 36 g of H 2 O 20 g of O 2 given 225 g H 2 O. 4 g of hydrogen reacts with 20 g of oxygen to form water. Join the 2 Crores Student community now. 4 g of hydrogen reacts with 20 g of oxygen to form water. 10 g of hydrogen molar mass 2 gmol 2 gmol 10 g 5 mol 64 of oxygen molar mass 32 gmol 32 gmol 64 g 2 mol.

When 3 0 G Of Carbon Is Burnt In 8 00 G Oxygen 11 00 G Of Carbon Dioxide Is Produced What Mass
| Title: When 3 0 G Of Carbon Is Burnt In 8 00 G Oxygen 11 00 G Of Carbon Dioxide Is Produced What Mass |
| Format: PDF |
| Number of Pages: 140 pages 4 G Of Hydrogen Reacts With 20g Of Oxygen |
| Publication Date: April 2021 |
| File Size: 6mb |
| Read When 3 0 G Of Carbon Is Burnt In 8 00 G Oxygen 11 00 G Of Carbon Dioxide Is Produced What Mass |
 |

How Many Grams Of Oxygen Are Required To Burn Pletely 570g Of Octane
| Title: How Many Grams Of Oxygen Are Required To Burn Pletely 570g Of Octane |
| Format: ePub Book |
| Number of Pages: 303 pages 4 G Of Hydrogen Reacts With 20g Of Oxygen |
| Publication Date: May 2020 |
| File Size: 3.4mb |
| Read How Many Grams Of Oxygen Are Required To Burn Pletely 570g Of Octane |
 |

80 G Of H2 Is Reacted With 80 G Of O2 To Form Water Find Out The Mass Of Water Obtained Which Substance Is The Limiting Reagent
| Title: 80 G Of H2 Is Reacted With 80 G Of O2 To Form Water Find Out The Mass Of Water Obtained Which Substance Is The Limiting Reagent |
| Format: ePub Book |
| Number of Pages: 166 pages 4 G Of Hydrogen Reacts With 20g Of Oxygen |
| Publication Date: July 2019 |
| File Size: 1.9mb |
| Read 80 G Of H2 Is Reacted With 80 G Of O2 To Form Water Find Out The Mass Of Water Obtained Which Substance Is The Limiting Reagent |
 |
Syzmrmgcnyoifm
| Title: Syzmrmgcnyoifm |
| Format: PDF |
| Number of Pages: 159 pages 4 G Of Hydrogen Reacts With 20g Of Oxygen |
| Publication Date: September 2018 |
| File Size: 1.6mb |
| Read Syzmrmgcnyoifm |
 |
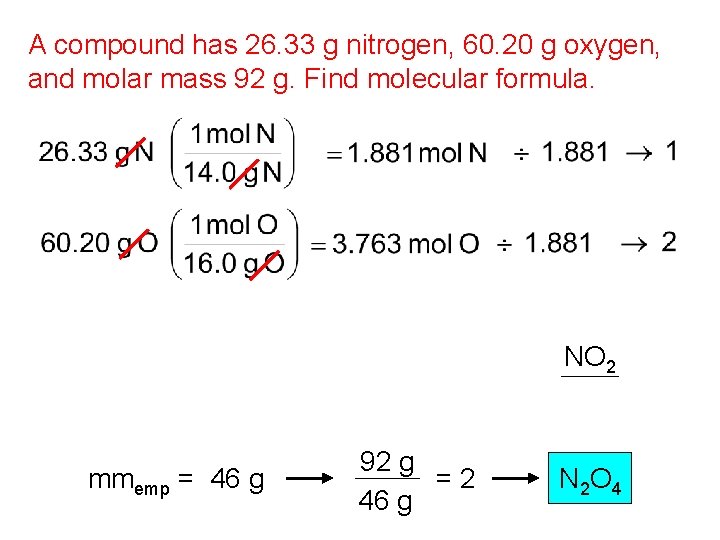
Spring Final Exam Stoichiometry Review Molar Mass The
| Title: Spring Final Exam Stoichiometry Review Molar Mass The |
| Format: eBook |
| Number of Pages: 309 pages 4 G Of Hydrogen Reacts With 20g Of Oxygen |
| Publication Date: January 2018 |
| File Size: 6mb |
| Read Spring Final Exam Stoichiometry Review Molar Mass The |
 |
How Much Water Would There Be If 5 Grams Of Oxygen And 5 Grams Of Hydrogen Were Mixed Quora
| Title: How Much Water Would There Be If 5 Grams Of Oxygen And 5 Grams Of Hydrogen Were Mixed Quora |
| Format: eBook |
| Number of Pages: 255 pages 4 G Of Hydrogen Reacts With 20g Of Oxygen |
| Publication Date: November 2021 |
| File Size: 2.8mb |
| Read How Much Water Would There Be If 5 Grams Of Oxygen And 5 Grams Of Hydrogen Were Mixed Quora |
 |

If We Have 4g Of Methane And 8g Of Oxygen Then Will It Conta Scholr
| Title: If We Have 4g Of Methane And 8g Of Oxygen Then Will It Conta Scholr |
| Format: eBook |
| Number of Pages: 130 pages 4 G Of Hydrogen Reacts With 20g Of Oxygen |
| Publication Date: June 2018 |
| File Size: 1.2mb |
| Read If We Have 4g Of Methane And 8g Of Oxygen Then Will It Conta Scholr |
 |

In The Chemical Reaction 2 H 2 G O2 G 2 H2o
| Title: In The Chemical Reaction 2 H 2 G O2 G 2 H2o |
| Format: ePub Book |
| Number of Pages: 180 pages 4 G Of Hydrogen Reacts With 20g Of Oxygen |
| Publication Date: September 2021 |
| File Size: 1.2mb |
| Read In The Chemical Reaction 2 H 2 G O2 G 2 H2o |
 |

1 4g H2 Reacts With 20g O2 To Form Water How Much Water Is Formed 2it Was Found That 380ml Of A Gas At 27 C And 800mm Of Hg Weighed 0455g
| Title: 1 4g H2 Reacts With 20g O2 To Form Water How Much Water Is Formed 2it Was Found That 380ml Of A Gas At 27 C And 800mm Of Hg Weighed 0455g |
| Format: PDF |
| Number of Pages: 195 pages 4 G Of Hydrogen Reacts With 20g Of Oxygen |
| Publication Date: December 2017 |
| File Size: 725kb |
| Read 1 4g H2 Reacts With 20g O2 To Form Water How Much Water Is Formed 2it Was Found That 380ml Of A Gas At 27 C And 800mm Of Hg Weighed 0455g |
 |

4 G H2 Reacts With 20 G O2 To Form Water How Much Water Is Formed
| Title: 4 G H2 Reacts With 20 G O2 To Form Water How Much Water Is Formed |
| Format: ePub Book |
| Number of Pages: 334 pages 4 G Of Hydrogen Reacts With 20g Of Oxygen |
| Publication Date: May 2017 |
| File Size: 800kb |
| Read 4 G H2 Reacts With 20 G O2 To Form Water How Much Water Is Formed |
 |

Calculate The Number Of Molecules In 4 G Of Oxygen
| Title: Calculate The Number Of Molecules In 4 G Of Oxygen |
| Format: eBook |
| Number of Pages: 293 pages 4 G Of Hydrogen Reacts With 20g Of Oxygen |
| Publication Date: February 2021 |
| File Size: 2.6mb |
| Read Calculate The Number Of Molecules In 4 G Of Oxygen |
 |
4 g of hydrogen reacts with 20 g of oxygen to form water. The mass of water formed is. 4 g of hydrogen reacts with 20 g of oxygen to form water.
Here is all you need to know about 4 g of hydrogen reacts with 20g of oxygen 4 g of hydrogen reacts with 20 g of oxygen to form water. Shop for cheap price 4 G Of Hydrogen Reacts With 20g Of Oxygen Price Low and Options of 4 G Of Hydrogen Reacts With 20g Of Oxygen from variety stores in usa. The mass of water formed is. Spring final exam stoichiometry review molar mass the 80 g of h2 is reacted with 80 g of o2 to form water find out the mass of water obtained which substance is the limiting reagent calculate the number of molecules in 4 g of oxygen when 3 0 g of carbon is burnt in 8 00 g oxygen 11 00 g of carbon dioxide is produced what mass if we have 4g of methane and 8g of oxygen then will it conta scholr how many grams of oxygen are required to burn pletely 570g of octane Now present oxygen is 20 g So O 2 will be the limiting reagent and H 2 O will be calculated from O 2 32 g of O 2 given 36 g of H 2 O 20 g of O 2 given 225 g H 2 O.




FOLLOW THE Charles Books Chapter AT TWITTER TO GET THE LATEST INFORMATION OR UPDATE
Follow Charles Books Chapter on Instagram to get the latest information or updates
Follow our Instagram